Respiratory Depressant Effects and Pharmacokinetics of Oral Mitragynine (Kratom) and Oxycodone in Rats
Marilyn A. Huestis, PhD1* , PinneyAssociates, Bethesda, MD, USA, Aaron Magnuson, PhD2 , Care Research, Fort Collins, CO, USA, Daniel Wang, BS1 , PinneyAssociates, Bethesda, MD, USA, Wanzhu Zhao, MA3 , iC42, University of Colorado, Aurora, CO, USA, Uwe Christians, MD, PhD3 , iC42, University of Colorado, Aurora, CO, USA, Jack E. Henningfield, PhD1 , PinneyAssociates, Bethesda, MD, USA
Learning Overview: Attendees will be able to better compare the respiratory depressant effects and relative toxicity of oral mitragynine and oxycodone.
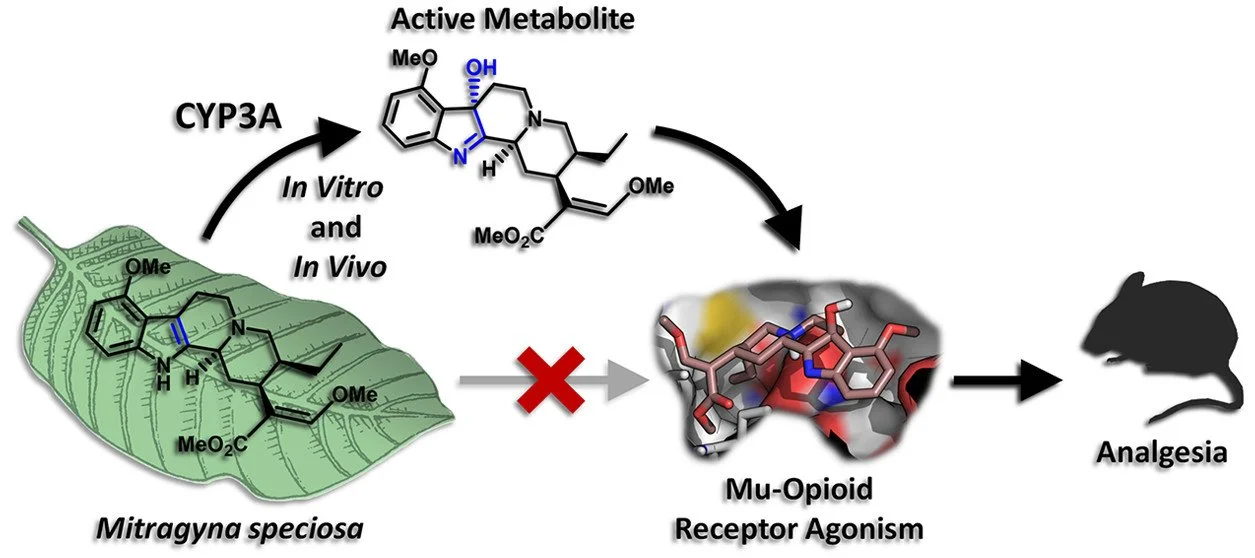
Impact on the Forensic Science Community: Lack of respiratory depression following highdose mitragynine in rats questions the appropriateness of designating kratom as cause of death in postmortem cases. Mitragynine, the primary active alkaloid in kratom and partial µ-opioid receptor agonist, has substantially different pharmacology from the full µ-opioid receptor agonists morphine and oxycodone. Mitragynine does not recruit β-arrestin for G-protein signaling, has activity at adrenergic receptors and a lower risk of respiratory depression, acute poisoning, death, and abuse-related effects. The kratom alkaloid, 7-hydroxy-mitragynine (7-OH-MG), constitutes <1.5% of the total alkaloid content, is more potent at the opioid receptor than mitragynine and a mitragynine metabolite. Mitragynine is not scheduled in the Controlled Substance Act; however, the FDA recommended scheduling of mitragynine in 2016, in part out of safety concerns, though the request was rescinded by the Assistant Secretary of Health in 2018. Mitragynine is taken by millions of people in Southeast Asia and the US as a mild stimulant and analgesic and to treat opioid dependence and withdrawal. In 2020, FDA recently recommended a rat model for evaluating drug respiratory depressant effects, utilizing oxycodone as a positive control. We employed this model to compare respiratory depression following 20, 40, 80, 240 and 400 mg/kg oral mitragynine and 6.75, 60 and 150 mg/kg oral oxycodone in rats. Oxycodone, mitragynine and 7-OH-mitragynine were quantified by LC-MS/MS pre-dose, 1, 2, 4, 6, 8 and 12 h after administration. Blood gases were determined at the same time points with the VetScan i-STAT Alinity V Handheld Analyzer. Clinical observations of opioid associated effects and toxicity were performed throughout. A significant decrease in oxygen saturation compared to baseline sO2 following the 60 and 150 mg/kg oxycodone doses was observed, with no significant differences in sO2 following 20, 40, 80, 240 and 400 mg/kg mitragynine over 12 h. Partial CO2 pressure change from baseline was significantly increased after 60 and 150 mg/kg oxycodone hydrochloride, but not following any mitragynine dose. Two animals receiving oxycodone, one at 60 and one at 150 mg/kg, died after dosing, and 66.7% and 100% of animals receiving these doses had abnormal clinical observations. One animal following 240mg/kg and multiple animals following 400 mg/kg mitragynine had abnormal clinical observations. Median blood oxycodone Cmax were 33.5, 39.9 and 131 ng/mL following 6.75, 60 and 150 mg/kg oxycodone, respectively, with Tmax at 1 h. Median mitragynine Cmax were 723, 1665, 2140, 6278 and 6341 ng/mL after 20, 40, 80, 240 and 400 mg/kg mitragynine, respectively, with Tmax generally at 1 h. 7-OH-mitragynine Cmax were 11.0, 17.4, 18.9, 32.0 and 62.5 ng/mL after 20, 40, 80, 240 and 400 mg/kg mitragynine, respectively, with Tmax at 1 h. 7-OH-mitragynine concentrations were approximately 100-fold lower than mitragynine concentrations in the same sample. Mitragynine doses as high as 400 mg/kg did not produce any clinically relevant or significantly measurable effects on respiration based on blood gas measurement, whereas 60 & 150 mg/kg oxycodone resulted in moderate to severe respiratory depression in rats. Similarly, there were no abnormal clinical observations after 20, 40 and 80 mg/kg, one after 240 mg/kg and multiple after 400 mg/kg oral mitragynine, while 60 and 150 mg/kg oxycodone produced many abnormal observations and two deaths. Conclusion: Oral mitragynine had little respiratory depression in rats following up to 400mg/kg doses, much higher doses than generally taken by humans, suggesting caution in attributing cause of death to mitragynine respiratory depression in postmortem deaths. _________________________________________________